
The chapter focuses on M13KO7 and its uses. Competition with both transforming and non-transforming plasmids indicates that each cell is capable of taking up many DNA molecules, and that the establishment of a transformation event is neither helped nor hindered significantly by the presence of multiple plasmids.read more read lessĪbstract: Publisher Summary This chapter discusses the production of single-stranded plasmid DNA. No significant variation is observed between competing DNAs of different source, complexity, length or form. Non-transforming DNAs compete consistent with mass. Relaxed and supercoiled plasmids transform with similar probabilities.

Transformation efficiency declines linearly with increasing plasmid size. These conditions include cell growth in medium containing elevated levels of Mg2+, and incubation of the cells at 0 degrees C in a solution of Mn2+, Ca2+, Rb+ or K+, dimethyl sulfoxide, dithiothreitol, and hexamine cobalt (III). A set of conditions is described under which about one in every 400 plasmid molecules produces a transformed cell. Thus, cloning the products as blunt-ended fragments requires enzymatic processing to remove of the 3' overhang using an enzyme with 3' to 5' exonuclease activity (11).read more read lessĪbstract: Factors that affect the probability of genetic transformation of Escherichia coli by plasmids have been evaluated. This nucleotide is almost exclusively an adenosine, due to the strong preference of the polymerase for dATP (9). Attempts to clone PCR products as blunt-ended fragments have been very inefficient, due to the template-independent terminal transferase activity of Taq polymerase, which results in the addition of a single nucleotide at the 3' end of the fragment (9, 10). Ligation-independent PCR cloning schemes (6, 7, 8) involve the addition of at least 12 bases to the 5' end of the primer, which can increase the cost of synthesis substantially when done routinely. Unfortunately, many restriction endonucleases fail to cleave when their recognition sequences are located within a few base pairs of the end of a DNA fragment (4, 5). Restriction endonuclease sites are often incorporated into the oligonucleotide primers used for amplification, so that cleavage of the product will create sticky ends that can theoretically be ligated to an equivalently cut vector (3). We have applied the procedure for the cloning of inter-ALU fragments from hybrid cell-lines and human cosmid clones.read more read lessĪbstract: Although the polymerase chain reaction (1,2) (PCR) can be used to produce a large amount of a specific DNA from a complex source, cloning the PCR products has not proven to be straightforward.
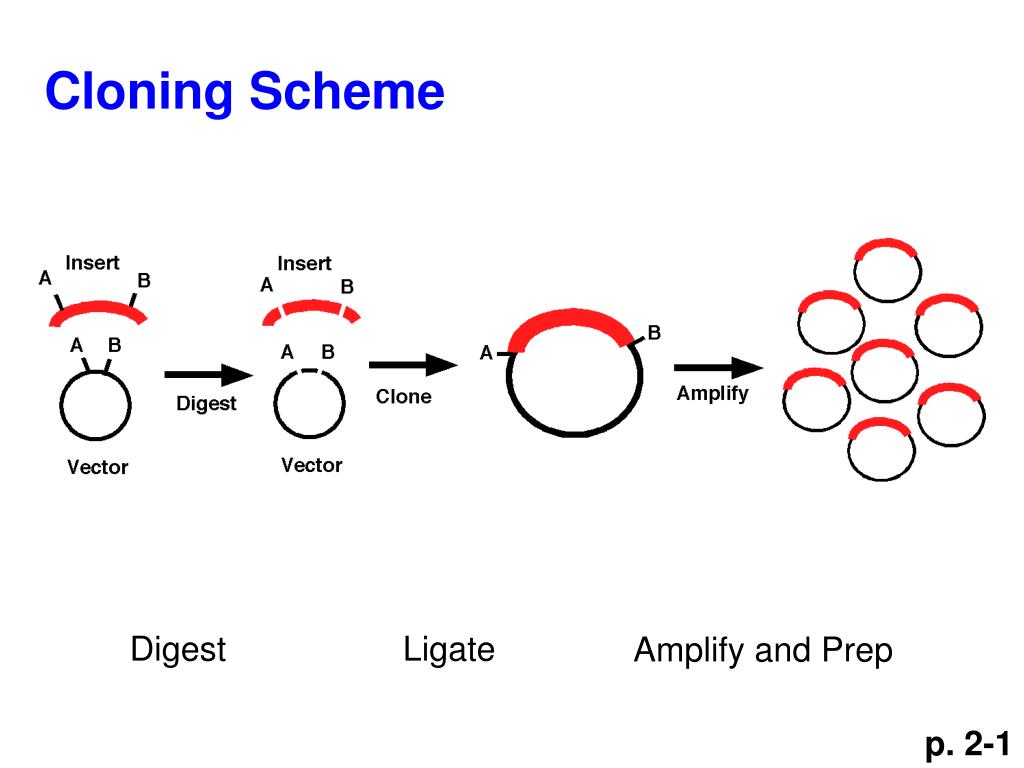
The resulting circular recombinant molecules do not require in vitro ligation for efficient bacterial transformation. Circularization can occur between vector molecules and PCR fragments as mediated by the 12-nt cohesive ends, but not in mixtures lacking insert fragments.
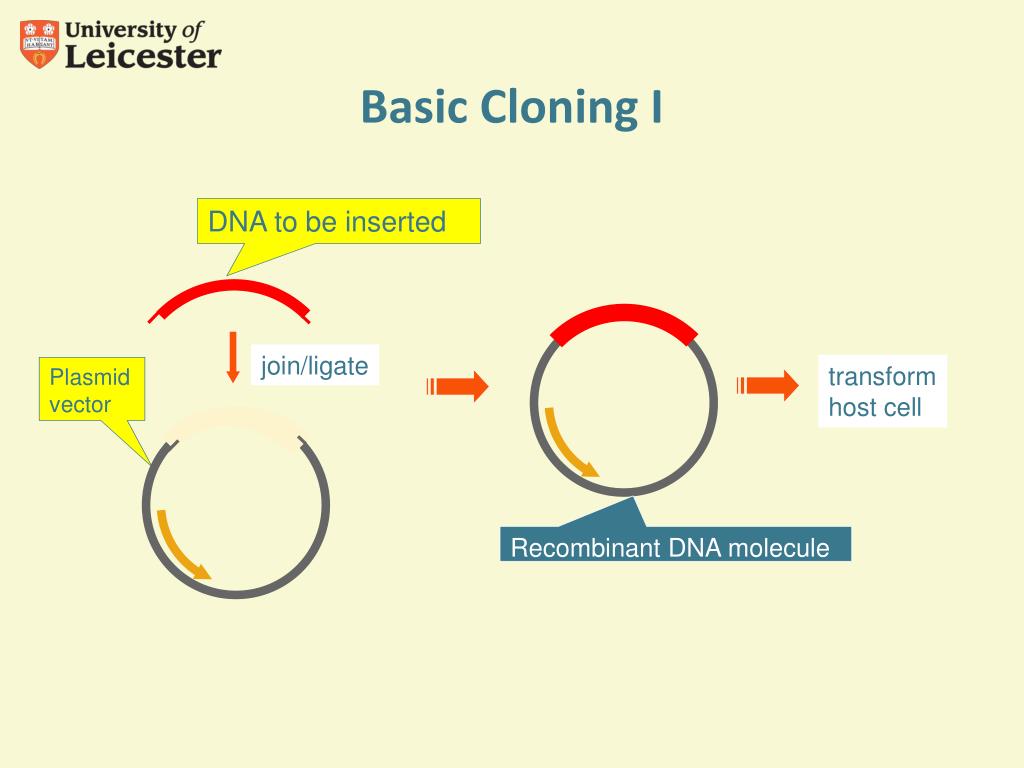
The vector oligos have additional 12-nt tails complementary to the tails used for fragment amplification, permitting the creation of ss-ends with T4 DNA polymerase in the presence of dCTP. Similarly, the entire plasmid vector is amplified with primers homologous to sequences in the multiple cloning site. The 3'-terminal sequence can be removed by the action of the (3'-5') exonuclease activity of T4 DNA polymerase in the presence of dGTP, leading to fragments with 5'-extending single-stranded (ss) tails of a defined sequence and length. As a result, the amplification products include 12-nt sequences lacking dGMP at their 3'-ends. The 5'-ends of the primers used to generate the cloneable PCR fragments contain an additional 12 nucleotide (nt) sequence lacking dCMP. The procedure does not require the use of restriction enzymes, T4 DNA ligase or alkaline phosphatase. Recombinants are generated between PCR products and a PCR-amplified plasmid vector. Abstract: A new procedure has been developed for the efficient cloning of complex PCR mixtures, resulting in libraries exclusively consisting of recombinant clones.


 0 kommentar(er)
0 kommentar(er)
